Energy Storage in Chemicals
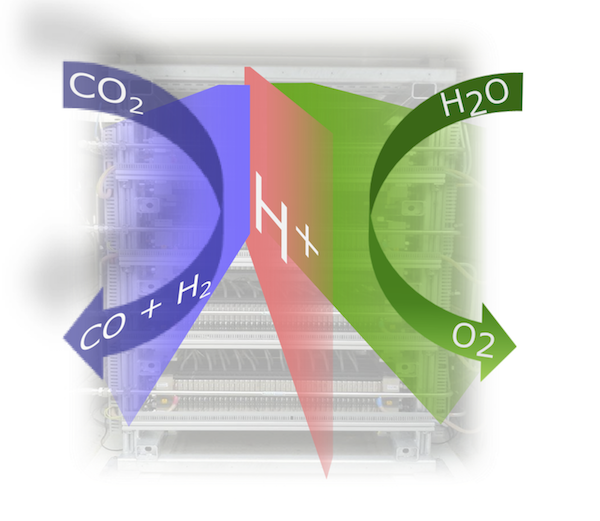
Electrical power produced by renewable energies can be chemically stored as hydrogen and other fuels via electrolysis. CO2 and H2O can be used in this context as feed in order to produce valuable chemical products, such as CO and hydrocarbon allowing storing excess energy by closing the CO2 cycle.
Within the external pageSwiss Competence Center for Energy Research (SCCER) Heat & Electricity Storagecall_made, one of the focus topics is related to energy storage in chemicals, e.g., hydrogen, formic acid, methanol or syn gas (CO/H2 mixtures). The latter products can be produced using both catalytic and electrocatalytic (co-electrolysis of CO2 and water) pathways. An economic analysis within the SCCER could demonstrate that specifically formic acid and electrochemically formed syn gas are products of choice using the co-electrolysis process under the premise that co-electrolysis cells can be operated at current densities of at least 0.2 A/cm2. Reaching these current densities, however, is a difficult task as typical known co-electrolysis cells rely on liquid CO2 being dissolved in water fed to the cell cathode, thereby being limited by the low solubility of CO2. Just recently, a novel co-electrolysis cell design was demonstrated by researchers from PSI’s Electrochemistry Laboratory, which allows to be operated at current densities up 1 A/cm2. This novel polymer membrane based cell design is directly fed by a humidified CO2 gas stream, overcoming any solubility issues present so far. Based on this cell design, it is now possible to scale-up the co-electrolysis process and to demonstrate the economic viability of electrochemical transformation of CO2 into chemical feedstock.
Link to the Project within SCCER Heat & Electricity Storage:
The ESC member involved in this project is Prof. Dr. Thomas J. Schmidt. The group of Prof. Schmidt is located at external pagePaul Scherrer Institutecall_made and the Laboratory of Physical Chemistry in D-CHAB and focuses on all aspects of electrochemical energy conversion and storage.
